Nutraceuticals Production and Capacity Outlook
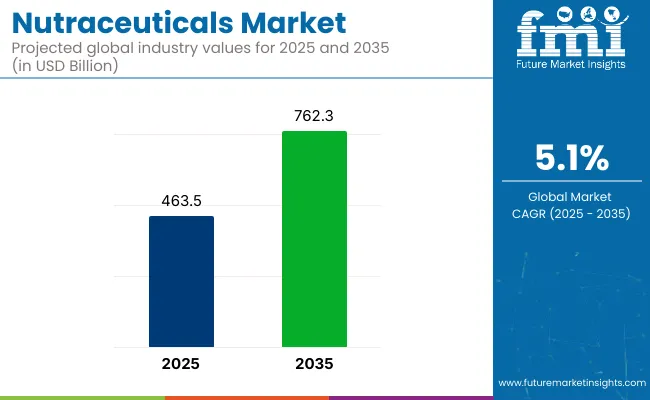
Global nutraceutical production capacity in 2026 is estimated at approximately 1.4 to 1.7 million tonnes on an active ingredient equivalent basis, reflecting sustained expansion driven by preventive health adoption, ageing populations and functional nutrition integration. Capacity growth is selective and formulation led, with investments concentrated in extraction, fermentation and encapsulation rather than bulk commodity scale up.
Production leadership remains concentrated in regions with strong dietary supplement manufacturing, biotechnology capability and regulatory clarity. North America leads global output supported by mature supplement consumption and contract manufacturing infrastructure. Asia Pacific expands rapidly through botanical extracts, traditional ingredients and fermentation based actives. Europe maintains a strong position in regulated, science backed nutraceuticals with emphasis on quality and traceability. Several regions remain import dependent due to limited bioactive processing capability.
Demand growth is reinforced by recurring consumption patterns and diversification across formats. Buyers prioritise ingredient consistency, bioavailability performance and compliance documentation.
Key Questions Answered
- How secure is sourcing of botanical and bioactive raw materials?
- How do regulatory approval timelines affect capacity expansion?
- How do formulation technologies influence production economics?
- How resilient is nutraceutical demand across economic cycles?
Nutraceutical Product Families That Define How Buyers Actually Use Them
Product Classification
- Vitamins and minerals
- Single nutrient formulations
- Multivitamin blends
- Fortification systems
- Botanical and herbal extracts
- Standardised plant extracts
- Traditional ingredient concentrates
- Functional phytonutrients
- Proteins and amino acids
- Sports nutrition ingredients
- Clinical nutrition components
- Functional food additives
- Lipids and fatty acids
- Omega 3 concentrates
- Phospholipids
- Specialty oils
- Probiotics and bioactives
- Live cultures
- Postbiotics
- Fermentation derived compounds
Vitamins and minerals account for the largest volume, while botanicals, probiotics and specialty bioactives drive higher value density and formulation complexity.
Key Questions Answered
- How do buyers differentiate commodity nutrients from specialty actives?
- How does standardisation affect formulation reliability?
- How do stability requirements vary by ingredient class?
- How do efficacy claims influence procurement decisions?
Nutraceutical Process Routes That Define Cost, Quality and Scale
Process Classification
- Extraction and concentration
- Solvent based extraction
- Supercritical processing
- Yield and purity optimisation
- Fermentation and biosynthesis
- Microbial production
- Precision fermentation
- Controlled bioactivity output
- Chemical synthesis and modification
- Vitamin synthesis
- Functional derivative production
- High purity refinement
- Encapsulation and delivery systems
- Spray drying
- Microencapsulation
- Bioavailability enhancement
Process selection prioritises bioactivity preservation, consistency and regulatory compliance. Advanced delivery technologies increasingly differentiate suppliers.
Key Questions Answered
- How sensitive nutraceutical cost is to raw material variability?
- How do processing methods affect bioavailability?
- How do quality controls influence scalability?
- How does process selection affect regulatory acceptance?
Nutraceutical End Use Spread Across Key Health Segments
End Use Segmentation
- Dietary supplements
- Tablets and capsules
- Powders and sachets
- Gummies and chewables
- Functional foods and beverages
- Fortified foods
- Ready to drink products
- Nutrition bars
- Sports and active nutrition
- Performance supplements
- Recovery formulations
- Endurance products
- Clinical and medical nutrition
- Specialised nutrition
- Elderly care formulations
- Therapeutic support products
Dietary supplements dominate consumption, while functional foods and sports nutrition drive diversification and innovation.
Key Questions Answered
- How do brands select ingredient formats by end use?
- How do dosage requirements affect formulation strategy?
- How do shelf life constraints shape product design?
- How do regulatory categories differ across applications?
Nutraceutical Regional Production Potential Assessment
North America
North America leads global production supported by contract manufacturing, regulatory frameworks and strong consumer demand.
Asia Pacific
Asia Pacific expands capacity through botanical extracts, fermentation based actives and traditional ingredient integration.
Europe
Europe focuses on science driven nutraceuticals, strict quality standards and traceability led production.
Latin America
Latin America shows growing potential tied to botanical diversity but remains limited in processing scale.
Middle East and Africa
These regions remain early stage with rising consumption but limited domestic production.
Key Questions Answered
- How do regional regulations influence product portfolios?
- How do import dependent regions manage supply risk?
- How does ingredient origin affect regional competitiveness?
- How do logistics affect ingredient stability?
Nutraceutical Supply Chain, Cost Drivers and Trade Patterns
The nutraceutical supply chain spans raw material cultivation or synthesis, processing, formulation and distribution to brand owners. Traceability and documentation are critical due to health related claims.
Key cost drivers include raw material availability, processing yield, encapsulation complexity and compliance expenditure. Trade flows connect ingredient rich regions with formulation and branding hubs.
Contracts emphasise specification adherence, documentation support and long term supply continuity.
Key Questions Answered
- How do raw material costs translate into finished ingredient pricing?
- How do quality audits affect supplier qualification?
- How do buyers benchmark bioactive potency?
- How do logistics and storage affect delivered quality?
Nutraceutical Ecosystem View and Strategic Themes
The ecosystem includes ingredient producers, contract manufacturers, supplement brands, food companies, healthcare professionals and regulators. Supply concentration reflects technical, regulatory and quality barriers.
Strategic themes include science backed claims, bioavailability enhancement, clean label positioning and supply chain transparency.
Deeper Questions Decision Makers Should Ask
- How secure is long term access to key bioactive ingredients?
- How diversified are sourcing strategies across regions?
- How exposed are portfolios to regulatory reinterpretation?
- How scalable are advanced delivery technologies?
- How robust are quality and traceability systems?
- How aligned are suppliers with clinical evidence requirements?
- How transparent are sustainability disclosures?
- How quickly can formulations adapt to new health trends?
Bibliography
- World Health Organization. (2024). Dietary supplements, nutrition guidelines, and safety considerations. WHO Press.
- Food and Agriculture Organization of the United Nations. (2024). Functional foods, bioactive ingredient sourcing, and food system nutrition trends. FAO.
- Organisation for Economic Co-operation and Development. (2023). Biotechnology, fermentation-based ingredients, and health-related chemical products. OECD Publishing.
Frequently Asked Questions
What is the estimated global nutraceutical production volume in 2025?
Global nutraceutical production in 2025 is estimated at approximately 1 to 2 million tonnes on an active ingredient equivalent basis, driven by dietary supplements and functional nutrition.
What are the biggest cost drivers shaping nutraceutical pricing?
Pricing is driven by raw material sourcing, processing complexity, encapsulation technology and regulatory compliance costs.
How do regulations affect nutraceutical availability?
Regulatory frameworks define permissible claims, ingredient approval and manufacturing standards, influencing product portfolios and investment decisions.
Why is bioavailability important in nutraceutical formulation?
Enhanced bioavailability improves efficacy at lower dosages, supporting differentiation and compliance with intake guidelines.
Which segments drive future nutraceutical growth?
Probiotics, botanical extracts, sports nutrition and healthy ageing formulations are expected to drive growth.